A Race Against Time: The CureDuchenne Clinic’s Remarkable Efforts Bring Life-Changing Gene Therapy to Young Boy on the Brink of Ineligibility

In an incredible story of determination and medical excellence, Hudson Sanford, a young patient at the CureDuchenne Clinic located at the Neurology & Neuromuscular Care Center in Denton, TX, is one of the earliest recipients of a groundbreaking gene therapy for Duchenne muscular dystrophy.
This milestone achievement came just three days before Hudson’s sixth birthday, a day that carried not only celebratory significance but also a deadline for treatment eligibility due to his age. Had Hudson turned 6, he would no longer have been eligible to receive this gene therapy. Dr. Diana Castro and the CureDuchenne Clinic rose to the occasion, defying time constraints and logistical challenges to offer hope to Hudson and his family.

The Shocking Diagnosis
Hudson’s journey began in August 2022 when he was almost 5 years old. Melanie and Phillip Sanford, his parents, received a diagnosis that would change their lives forever. Hudson was diagnosed with Duchenne muscular dystrophy, a devastating neuromuscular disease. “We had no idea what it was,” shared Melanie Burton-Sanford, recalling the moment they were confronted with this overwhelming reality.
A Glimmer of Hope
Hope flickered back into their lives when they heard about Elevidys, the gene therapy from Sarepta Therapeutics that was approved by the FDA for boys ages 4-5 in June 2023. The approval made headlines as the first gene therapy approved by the FDA to treat Duchenne. However, Melanie and Phillip were uncertain about Hudson’s eligibility for this cutting-edge therapy as his sixth birthday approached in August 2023.
Consultations with medical professionals seemed to reinforce their concerns. A doctor at a larger medical institution informed them that the logistics required for gene therapy would delay treatment for at least six months. That delay would have made Hudson ineligible, so time was of the essence.

A Pathway Unveiled
Fortunately, Melanie and Phillip Sanford were put in contact with Dr. Diana Castro and the CureDuchenne Clinic on August 4, marking a pivotal moment in their journey. They would have to travel from Arizona to Texas for a consultation, but they were willing to cross any distance to help their child. They knew the risks of the treatment, but doing nothing had one certain outcome – his muscles would continue to deteriorate from the degenerative disease.
Just three days after speaking with Dr. Castro, they had their first appointment, which brought them closer to the possibility of treatment. The clock was ticking on Hudson’s eligibility, but the clinic was prepared to move mountains to bring this treatment to Hudson and his family.
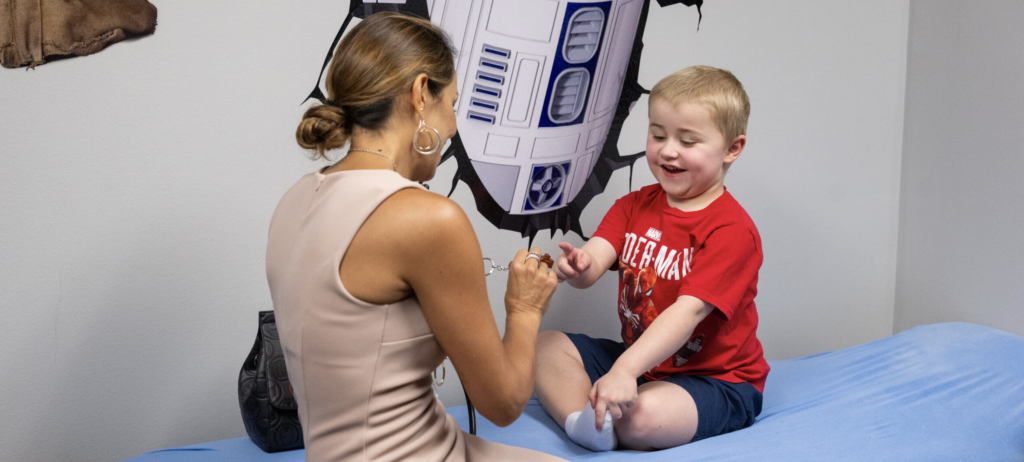
On the brink of Hudson’s eligibility deadline and just 11 days after their first appointment, the Sanford family received an extraordinary gift—the chance for Hudson to receive the gene therapy. Dr. Castro and the CureDuchenne Clinic worked tirelessly to make this happen, culminating in a milestone for both the clinic and the Sanford family when Hudson was dosed with Elevidys on August 18, 2023. “Dr. Castro, the CureDuchenne Clinic, and everyone who came together to make this happen, are truly amazing. We are so grateful,” said Phillip Sanford.
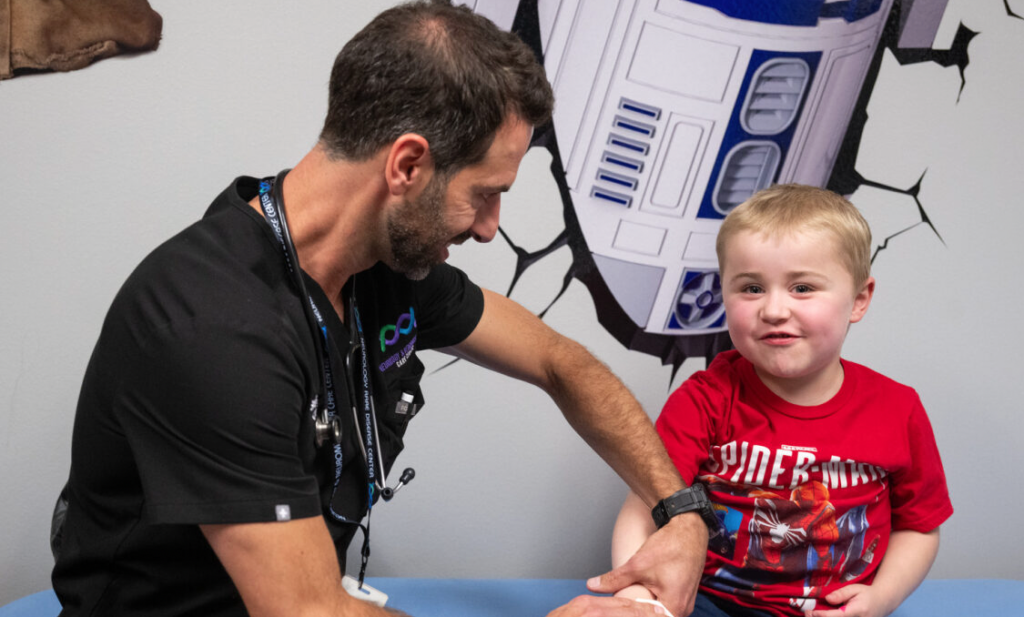
The Sanfords are also grateful to their family that supported them, including grandparents who accompanied Hudson to appointments, drove out to Texas, and stood by their side. Sarepta also played a key role in ensuring the Sanfords had access to treatment and support. Melanie Sanford reflected, “From the journey of his diagnosis to now, I just can’t believe we had this opportunity. It has restored my faith in humanity – even people who we don’t even know were helping us.”

Bringing Access to Families with Duchenne
Hudson’s story is not only a testament to the power of medical progress but also a source of hope for families navigating the challenging landscape of rare diseases. Without the relentless dedication of Dr. Diana Castro and the CureDuchenne Clinic, this avenue of treatment might have remained inaccessible.
“Time is of the essence in cases like Hudson’s. Our ability to get him into our clinic, get all approvals necessary, and administer the medication underscores the importance of a streamlined process and access to quality care for all who need it,” said Dr. Castro. “Delivering care like this is exactly why I entered the field of medicine.”
Debra Miller, Founder and CEO of CureDuchenne, highlighted the significant role of the CureDuchenne Clinic and CureDuchenne’s commitment to expanding treatment for individuals with Duchenne: “Dr. Castro and the CureDuchenne Clinic rose to the occasion, defying time constraints and logistical challenges to offer hope to Hudson and his family. It’s a testament to our commitment to providing transformative treatments and unwavering support for those navigating the challenges of Duchenne. This marks a tremendous milestone, but our work is far from done. There are still many individuals waiting for treatments and in need of high-quality care, and we will continue to support and invest in promising research and programs to bring treatments to everyone in need.”




