Updates on CureDuchenne Gene Therapy Initiative
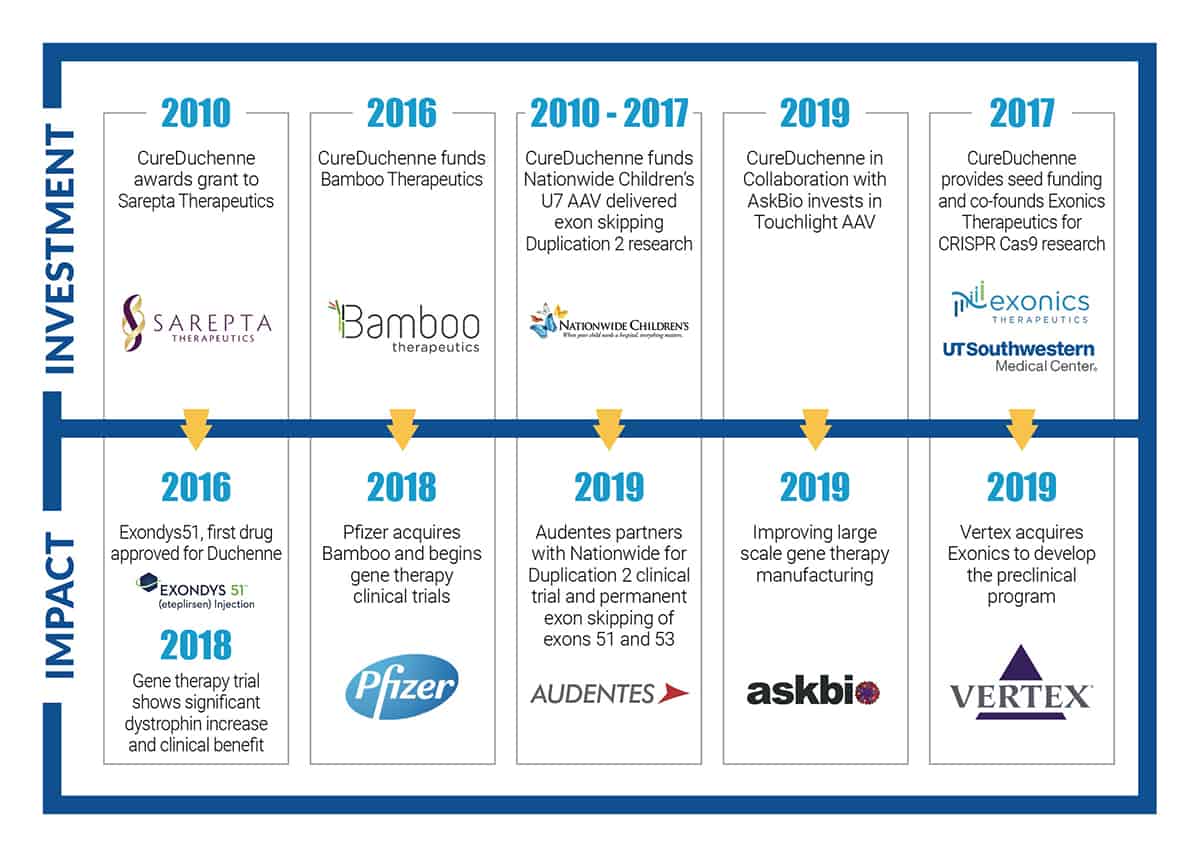
We are excited to report the progress of CureDuchenne’s Gene Therapy Initiative. From our investment, as early as 2010, in Nationwide Children’s permanent duplication2 skipping program, to what has become Pfizer’s Duchenne gene therapy trial, to founding the first Duchenne gene editing company, Exonics (recently acquired by Vertex), to investing in crucial AAV manufacturing in collaboration with AskBio, CureDuchenne is leading gene therapy investments among Duchenne organizations.
This is great news for the Duchenne community. As the world looks to gene therapy as a cure for genetic diseases like Duchenne, developing a therapy that works on a large scale is complicated and mass production of AAV is a significant obstacle. To date, no other Duchenne organization has invested in this critical step that is necessary to realize the promise of gene therapy for our loved ones. Our investment in Touchlight/AskBio was to specifically address the hurdles of AAV manufacturing.
Two additional and substantial obstacles that stand in the way of all Duchenne patients having access are pre-existing antibodies, and the option to re-dose, should the need occur.
While gene therapy development is moving quickly, all three of these obstacles must be addressed. CureDuchenne is seeking out and investing in the most promising research projects that will solve these problems. We are funding projects in gene therapy, gene editing, delivery systems and manufacturing – the new frontier to ensure all patients have access to a cure for Duchenne.
My son Hawken is now 22. The likelihood that gene therapy will work for him is small, but we will not give up hope that everyone affected by Duchenne will have a treatment.
Please review the chart below illustrating our gene therapy projects and join us as we continue our mission.
With gratitude,
Debra Miller